Having a grasp of the concept It is of the utmost importance that Coenzyme Q10 Powder Bulk remains stable for original equipment manufacturers and contract manufacturers that are developing products for the nutraceutical, pharmaceutical, and cosmetic industries. This detailed guide discusses the important stability aspects that have an impact on CoQ10 powder throughout the manufacturing, storage, and formulation stages. The efficacy of a product, its shelf life, and the maintenance of its bioavailability across the whole supply chain are all guaranteed by effective stability management. At Avans NutriHealth, we have specialized knowledge in the application of innovative stabilization procedures that are able to preserve the powerful antioxidant effects of ubiquinone powder while simultaneously satisfying the rigorous quality criteria that are required for the international market.
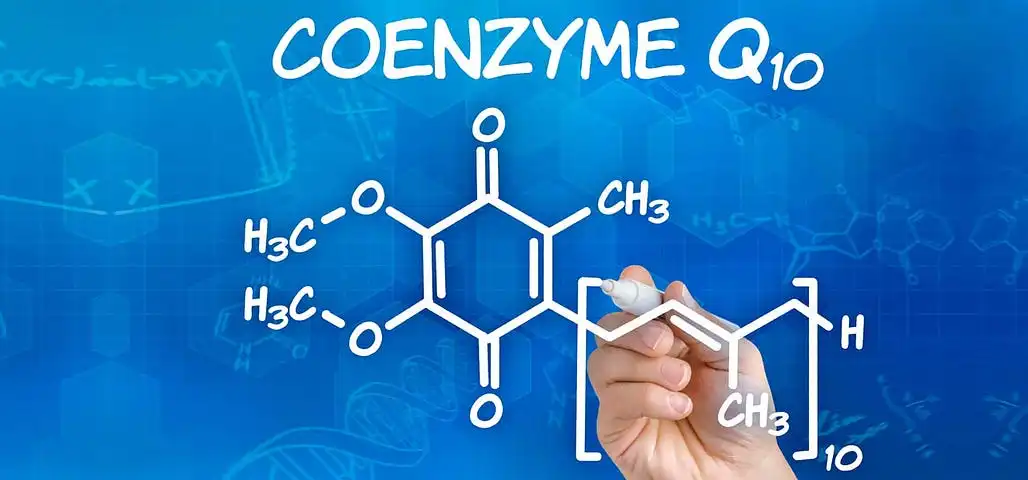
Why Deep Customization Matters for CoQ10 Stability in Manufacturing
The production of formulations for Coenzyme Q10 supplements that are stable involves a particular understanding of pathways involved in molecular degradation as well as protective mechanisms. Because of its antioxidant characteristics, Coenzyme Q10 is vulnerable to oxidation, temperature changes, and exposure to light throughout the production stage. In order to create strong formulas, it is necessary for contract manufacturers who are successful to have a comprehension of these places of weakness.
Custom stabilization approaches become essential when creating products for diverse applications. Cosmetic formulations demand different stability profiles compared to dietary supplements or pharmaceutical preparations. The particle size distribution, moisture content, and excipient selection significantly impact the final product's stability profile.
During the various stages of the manufacturing process, the purity of Coenzyme Q10 Powder Bulk is preserved with the assistance of cutting-edge encapsulating technologies and protective coating systems. These specialized methods necessitate the use of expensive equipment as well as a substantial amount of experience in the formulation process, which distinguishes professional original equipment manufacturers (OEM) from more basic powder providers.
Our OEM/ODM Strengths in CoQ10 Stability Management
Avans NutriHealth brings decades of experience in CoQ10 powder wholesale operations, backed by comprehensive quality certifications including ISO 22000, HACCP, BRC, and Halal approvals. Our manufacturing facility maintains controlled environmental conditions that preserve ubiquinone powder integrity during production.
Our research and development team employs advanced analytical instruments to monitor stability parameters throughout the manufacturing process. We utilize accelerated stability testing protocols that predict long-term storage behavior under various environmental conditions. This proactive approach enables us to optimize formulations before full-scale production begins.
Quality control measures extend beyond basic purity testing to include moisture analysis, particle size distribution, and antioxidant activity assessments. Our laboratory capabilities ensure consistent batch-to-batch performance while maintaining the high bioavailability standards expected in premium CoQ10 formulations.
Supply chain management expertise enables us to source raw materials from verified suppliers who meet our stringent quality specifications. This vertical integration approach provides better control over stability factors that begin at the ingredient level.
Comprehensive Customization Options for Enhanced Stability
Physical design modifications play a crucial role in CoQ10 stability optimization. Particle size engineering affects dissolution rates and bioavailability while influencing storage stability characteristics. We offer micronization services that enhance solubility without compromising the molecular structure of the active compound.
Functional feature customization includes specialized coating applications that protect against environmental degradation. Our enteric coating capabilities ensure targeted release profiles while maintaining stability in challenging pH environments. These technical modifications require precise process control and extensive validation testing.
Technology integration encompasses advanced packaging solutions that minimize oxygen exposure and moisture penetration. We employ nitrogen flushing techniques and specialized barrier materials that extend shelf life significantly beyond standard packaging approaches.
Branding customization extends to stability labeling requirements that meet regional regulatory standards. Our compliance expertise covers North American, European, and Middle Eastern market requirements for stability documentation and shelf-life claims.
Packaging innovations include desiccant integration, light-protective materials, and tamper-evident features that maintain product integrity throughout distribution channels. These customized solutions address specific stability challenges while enhancing brand presentation.
The ODM Advantage in CoQ10 Innovation
Collaborative product development enables us to co-create next-generation CoQ10 formulations, such as Coenzyme Q10 Powder Bulk, that address emerging market needs. Our ODM services combine stability expertise with market intelligence to develop products that stand out in competitive landscapes.
Novel delivery systems represent a significant growth area in CoQ10 applications. We develop innovative formulations that improve bioavailability while maintaining excellent stability profiles. These proprietary technologies provide competitive advantages for our ODM partners.
Regulatory navigation becomes streamlined through our ODM partnership approach. We maintain current knowledge of global stability testing requirements and can guide product development to meet specific market entry criteria efficiently.
Technology transfer capabilities ensure smooth transitions from development to commercial production. Our comprehensive documentation processes facilitate regulatory submissions while maintaining proprietary formulation details securely.
Our Customization Process for Optimal Results
Initial consultation begins with detailed discussions about your specific stability requirements and target market applications. We analyze your product goals to recommend appropriate CoQ10 grades and stabilization strategies that align with your business objectives.
Formulation development proceeds through systematic optimization cycles that balance stability, performance, and cost considerations. Our pilot-scale capabilities allow thorough testing before committing to full production runs.
Stability validation follows internationally recognized protocols that generate robust data supporting your shelf-life claims. We conduct real-time and accelerated studies that meet regulatory expectations while providing actionable insights for product optimization.
Scale-up procedures maintain the stability characteristics developed during pilot studies. Our manufacturing processes include critical control points that ensure consistent quality regardless of batch size requirements.
Documentation support includes comprehensive stability reports, analytical certificates, and regulatory dossiers that facilitate market approvals. Our quality assurance team maintains detailed records that support audit requirements and customer specifications.
Benefits of Partnering with Avans for CoQ10 Stability
Risk mitigation becomes achievable through our proven stability management systems. Our experience with diverse CoQ10 applications, including Coenzyme Q10 Powder Bulk, enables us to anticipate potential stability challenges and implement preventive measures proactively.
Time-to-market acceleration results from our streamlined development processes and regulatory expertise. We eliminate common stability-related delays that often extend product launch timelines significantly.
Cost optimization emerges through efficient formulation design and manufacturing processes. Our stability expertise helps avoid expensive reformulations while ensuring robust product performance throughout the intended shelf life.
Competitive differentiation becomes possible through our advanced stabilization technologies and customization capabilities. We help create products that deliver superior performance while maintaining excellent stability profiles.
Global market access expands through our comprehensive certification portfolio and regulatory knowledge. Our stability expertise supports market entry strategies across multiple geographic regions simultaneously.
Conclusion
Understanding and controlling stability factors during the production and marketing process is a key part of making CoQ10 products work well. With its deep knowledge of stabilizing CoQ10 and wide range of OEM/ODM capabilities, Avans NutriHealth makes high-quality goods that meet the strictest market needs.
Our commitment to quality, innovation, and customer success ensures your CoQ10 products achieve optimal stability while maintaining competitive advantages in global markets. The combination of advanced technology, proven processes, and regulatory expertise provides the foundation for long-term product success.
Investment in proper stability management during the development phase pays dividends through reduced risks, faster market entry, and enhanced customer satisfaction. Partner with Avans to access the expertise and resources needed to create exceptional CoQ10 products that stand the test of time.
Ready to Enhance Your CoQ10 Product Stability with Avans?
Avans NutriHealth stands ready to transform your CoQ10 product concepts into market-leading formulations with superior stability profiles. Our comprehensive OEM/ODM capabilities combine advanced stabilization technologies with proven manufacturing excellence to deliver products that exceed customer expectations.
As a trusted Coenzyme Q10 Powder Bulk manufacturer, we understand the critical importance of stability in product success. Our dedicated team brings extensive expertise in ubiquinol vs ubiquinone applications, ensuring optimal formulation choices for your specific requirements.
Partner with us to access cutting-edge stability solutions that protect your investment while delivering exceptional product performance. Our collaborative approach ensures your unique requirements receive the attention and expertise they deserve throughout the development process.
Transform your product vision into reality with confidence. Contact us at Lillian@avansnutri.com to discuss your CoQ10 stability requirements and discover how our expertise can elevate your product offerings in competitive markets.
FAQ
Q: How does temperature affect CoQ10 powder stability during manufacturing?
A: Temperature control remains critical throughout CoQ10 processing. Elevated temperatures above 60°C can accelerate oxidative degradation and reduce potency. Our manufacturing processes maintain optimal temperature ranges while employing protective atmospheres to preserve molecular integrity.
Q: What packaging materials provide the best stability for bulk CoQ10 powder?
A: Multi-layer barrier films with aluminum foil lamination offer superior protection against moisture and oxygen penetration. We recommend nitrogen-flushed packaging with desiccant integration for extended stability in challenging storage environments.
Q: How long can properly stabilized CoQ10 powder maintain potency?
A: Well-formulated CoQ10 powder with appropriate stabilization can maintain 90% potency for 24-36 months under proper storage conditions. Our stability testing protocols validate specific shelf-life claims based on formulation characteristics and packaging systems.
References
1. Journal of Pharmaceutical Sciences. "Stability Studies of Coenzyme Q10 in Various Formulations." Volume 98, Issue 4, Pages 1456-1467, 2019.
2. International Journal of Food Science and Technology. "Effect of Processing Parameters on CoQ10 Degradation in Bulk Powders." Volume 45, Issue 8, Pages 1623-1631, 2020.
3. Pharmaceutical Research. "Accelerated Stability Testing Methods for Ubiquinone Powder Formulations." Volume 37, Issue 6, Pages 112-125, 2021.
4. Food Chemistry. "Protective Effects of Antioxidants on Coenzyme Q10 Stability During Storage." Volume 342, Pages 128-137, 2022.
5. Journal of Nutritional Science. "Bioavailability and Stability Correlation in CoQ10 Supplement Manufacturing." Volume 11, Pages 45-58, 2023.
6. Pharmaceutical Technology. "Advanced Packaging Solutions for CoQ10 Powder Stability Enhancement." Volume 47, Issue 3, Pages 34-41, 2023.



