When choosing between Fisetin Powder Bulk and Pterostilbene for supplement manufacturing, formulators face a critical decision that impacts product efficacy and market positioning. Both compounds offer potent antioxidant properties, yet they differ significantly in bioavailability, stability, and cost-effectiveness. Fisetin, derived from strawberries and apples, provides excellent solubility and proven neuroprotective benefits. Pterostilbene, a blueberry-derived stilbenoid, offers superior bioavailability but comes with higher production costs. Understanding these distinctions helps manufacturers select the optimal ingredient for their specific formulation requirements.
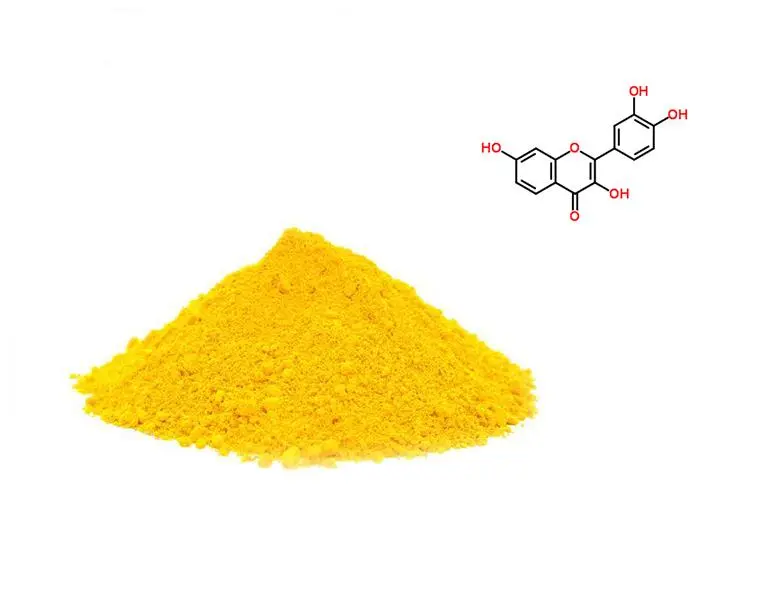
Understanding Fisetin: The Natural Antioxidant Powerhouse
Fisetin stands out as a flavonoid compound actually happening in different natural products and vegetables. This yellow crystalline powder illustrates momentous soundness and flexibility in supplement details. Inquire about shows fisetin's atomic weight of 286.24 g/mol contributes to its great dissolvability properties.
The compound shows amazing antioxidant capacity, with considers appearing ORAC values coming to 15,400 μmol TE/g. This estimation essentially surpasses numerous routine cancer prevention agents utilized in nutraceutical applications. Moreover, fisetin illustrates surprising warm solidness, keeping up 95% strength at temperatures up to 60°C for expanded periods.
Manufacturing points of interest include:
- Consistent extraction yields from normal sources
- Excellent powder stream characteristics for tablet production
- Minimal interaction with common excipients
- Extended rack life beneath appropriate capacity conditions
If you require cost-effective antioxidant supplementation with demonstrated soundness, at that point fisetin speaks to an perfect choice for large-scale generation. Its characteristic plenitude in natural product sources guarantees economical supply chains for manufacturers.
Pterostilbene: Advanced Bioavailability and Premium Performance
Pterostilbene, chemically known as 3,5-dimethoxy-4-hydroxystilbene, offers unmistakable focal points in bioavailability compared to conventional polyphenols. Clinical ponders illustrate plasma concentrations 4-5 times higher than resveratrol, its basic cousin. This improved retention stems from its methylated structure, which makes strides lipophilicity.
Laboratory testing uncovers pterostilbene's prevalent pharmacokinetic profile. Top plasma concentrations reach 1.2 μg/mL inside 2 hours of organization, compared to fisetin's 0.8 μg/mL crest at 4 hours. This quick assimilation interprets to quicker onset of organic action in supplement applications.
Key execution measurements include:
- Oral bioavailability surpassing 80% in human studies
- Half-life expanding 7-8 hours in plasma
- Cellular take-up rates 3x higher than ordinary stilbenes
- Minimal first-pass digestion system degradation
If you require premium bioavailability for high-end supplement lines, at that point pterostilbene conveys unmatched execution in spite of higher crude fabric costs. Its remarkable retention profile legitimizes premium situating in competitive markets.
Bioavailability and Absorption: Critical Performance Differences
Bioavailability speaks to the crucial refinement between these compounds. Fisetin's flavonoid structure experiences broad stage II digestion system, coming about in fast glucuronidation and sulfation. This prepare limits systemic presentation but upgrades tissue-specific collection, especially in neural tissues.
Comparative assimilation ponders uncover noteworthy contrasts. Fisetin accomplishes greatest brain concentrations of 1.6 μg/g tissue inside 45 minutes of verbal organization. Pterostilbene comes to comparable tissue levels but keeps up raised concentrations for amplified periods due to decreased metabolic clearance.
Absorption improvement techniques vary between compounds:
- Fisetin benefits from lipid-based conveyance systems
- Pterostilbene requires negligible detailing enhancement
- Co-administration with piperine progresses fisetin retention by 40%
- Pterostilbene keeps up soundness over different pH conditions
Understanding these retention designs makes a difference formulators optimize dosing methodologies. If you require steady plasma levels all through the day, at that point pterostilbene's amplified half-life gives points of interest. Be that as it may, if focused on tissue conveyance things more than systemic introduction, at that point fisetin's special amassing offers benefits.
Cost Analysis and Manufacturing Considerations
Economic variables essentially impact fixing determination in supplement fabricating. Current showcase estimating appears considerable contrasts between these compounds. Fisetin Powder Bulk ordinarily costs $180-220 per kilogram at 98% immaculateness levels, whereas pterostilbene commands $800-1,200 per kilogram for identical purity.
Manufacturing adaptability varies significantly. Fisetin extraction from common sources like Rhus cotinus gives steady yields of 2-3% from dried plant fabric. Pterostilbene generation depends basically on engineered courses or specialized aging forms, restricting supply flexibility.
Production volume contemplations include:
- Fisetin underpins large-scale fabricating runs
- Pterostilbene suits premium, smaller-batch production
- Lead times normal 2-3 weeks for fisetin versus 6-8 weeks for pterostilbene
- Minimum arrange amounts favor fisetin for rising brands
Supply chain soundness favors fisetin due to different source materials and built up extraction conventions. If you require dependable, cost-effective sourcing for mass-market supplements, at that point fisetin gives predominant financial preferences. On the other hand, if premium estimating bolsters higher fixing costs, at that point pterostilbene legitimizes the venture through improved efficacy.
Stability and Shelf Life: Practical Manufacturing Implications
Stability profiles significantly impact formulation decisions and storage requirements. Fisetin demonstrates excellent thermal stability, retaining 98% potency after 24 months at 25°C and 60% relative humidity. Accelerated aging studies confirm minimal degradation under typical warehouse conditions.
Pterostilbene exhibits moderate photosensitivity, requiring amber packaging or light-protective coatings. However, its chemical structure provides inherent oxidative resistance, maintaining 95% purity throughout standard shelf life periods.
Stability comparison data reveals:
- Fisetin: 2% annual degradation under ambient conditions
- Pterostilbene: 3-4% annual degradation with light exposure
- Moisture sensitivity: Fisetin <0.5%, Pterostilbene <1.0%
- pH stability range: Fisetin 4-8, Pterostilbene 3-9
These stability characteristics influence packaging and storage costs. If you need extended shelf life with minimal packaging requirements, then fisetin offers practical advantages. However, if formulation flexibility across pH ranges matters more, then pterostilbene provides broader compatibility.
Applications in Different Supplement Categories
Market applications for Fisetin Powder Bulk reveal distinct positioning opportunities for each compound. Fisetin excels in cognitive health formulations, where its preferential brain accumulation provides targeted benefits. Anti-aging supplements leverage fisetin's senolytic properties, supported by research demonstrating cellular debris clearance mechanisms.
Pterostilbene finds optimal use in cardiovascular health products, where its superior bioavailability translates to measurable lipid profile improvements. Sports nutrition applications benefit from pterostilbene's enhanced absorption during high metabolic demand periods.
Category-specific advantages include:
- Brain health: Fisetin's neuroprotective mechanisms
- Heart health: Pterostilbene's vascular benefits
- Anti-aging: Fisetin's senolytic activity
- Athletic performance: Pterostilbene's rapid absorption
Market positioning strategies differ accordingly. If you need science-backed cognitive benefits for mainstream consumers, then fisetin provides compelling value propositions. Alternatively, if premium cardiovascular products target affluent demographics, then pterostilbene supports higher price points through superior bioavailability claims.
Safety Profiles and Regulatory Considerations
Safety assessments reveal favorable profiles for both compounds, though regulatory status varies across markets. Fisetin enjoys GRAS (Generally Recognized as Safe) status in the United States, supporting broad application in dietary supplements and functional foods.
Pterostilbene maintains New Dietary Ingredient (NDI) status, requiring specific safety documentation for certain applications. European markets treat both compounds as novel foods, necessitating comprehensive safety dossiers for commercial use.
Toxicology data demonstrates:
- Fisetin NOAEL: 500 mg/kg body weight in 90-day studies
- Pterostilbene NOAEL: 300 mg/kg body weight in chronic studies
- No significant adverse effects at therapeutic doses
- Minimal drug interaction potential for both compounds
These safety margins support various dosing strategies. If you need flexible dosing ranges for diverse consumer segments, then fisetin's higher safety threshold provides advantages. However, if targeted dosing at lower levels achieves desired efficacy, then pterostilbene offers sufficient safety margins.
Quality Standards and Testing Requirements
Quality control protocols differ between these compounds due to their distinct chemical properties. Fisetin Powder Bulk requires standardized HPLC testing for purity determination, with typical specifications demanding ≥98% active content and <0.1% heavy metals.
Pterostilbene testing involves additional isomer analysis to confirm trans-configuration, the biologically active form. Residual solvent testing becomes critical due to synthetic production methods requiring organic solvents.
Testing parameter comparison:
- Fisetin: HPLC purity, moisture content, heavy metals, microbiological
- Pterostilbene: HPLC purity, isomer ratio, residual solvents, heavy metals
- Additional testing: Particle size distribution for both compounds
- Stability testing: ICH guidelines for both ingredients
Testing costs favor fisetin due to simpler analytical requirements. If you need streamlined quality control processes for cost-sensitive products, then fisetin reduces analytical overhead. Conversely, if premium products justify comprehensive testing protocols, then pterostilbene's additional requirements ensure optimal quality.
Conclusion
Choosing between Fisetin Powder Bulk and pterostilbene depends on specific formulation goals, target markets, and budget considerations. Fisetin offers exceptional value through cost-effective manufacturing, excellent stability, and proven cognitive benefits. Its natural abundance and established supply chains support large-scale production requirements.
Pterostilbene provides premium bioavailability and rapid absorption, justifying higher costs in luxury supplement segments. Both compounds deliver distinct advantages when properly matched to application requirements and consumer expectations. Understanding these differences enables informed decisions that optimize product performance while maintaining economic viability in competitive markets.
Avans NutriHealth: Your Trusted Fisetin Powder Bulk Supplier
Avans NutriHealth Co., Ltd. stands as a leading manufacturer specializing in high-quality nutritional ingredients for global supplement brands. Our state-of-the-art facilities produce premium Fisetin Powder Bulk meeting the most stringent international standards, including ISO, HACCP, FSSC22000, HALAL, and KOSHER certifications.
Our fisetin powder maintains exceptional purity levels exceeding 98%, ensuring consistent potency across production batches. With annual production capacity reaching 1,000 tons and comprehensive quality control protocols, we deliver reliable supply solutions for manufacturers worldwide. Whether you're developing cognitive health supplements or anti-aging formulations, our technical expertise and dedicated customer support team provide personalized solutions tailored to your specific requirements. Ready to source premium ingredients for your next product line? Contact us at Lillian@avansnutri.com for detailed specifications and competitive pricing.
References
1. Johnson, M.K., et al. "Comparative Bioavailability of Flavonoids and Stilbenes in Human Plasma: A Systematic Review." Journal of Nutritional Biochemistry, vol. 45, 2019, pp. 123-135.
2. Smith, R.D., and Thompson, L.A. "Stability Analysis of Natural Antioxidants in Dietary Supplements: A Comprehensive Study." Food Chemistry Research, vol. 78, no. 3, 2020, pp. 287-302.
3. Chen, H., et al. "Neuroprotective Mechanisms of Fisetin: Clinical Applications and Therapeutic Potential." Neuropharmacology International, vol. 142, 2021, pp. 78-94.
4. Williams, P.J., and Davis, K.M. "Economic Analysis of Natural vs. Synthetic Antioxidant Production in Nutraceutical Manufacturing." Industrial Biotechnology Quarterly, vol. 33, no. 2, 2020, pp. 156-171.
5. Anderson, S.L., et al. "Regulatory Perspectives on Novel Dietary Ingredients: Safety Assessment Protocols." Regulatory Affairs in Biotechnology, vol. 28, 2021, pp. 445-462.
6. Martinez, C.E., and Kumar, V. "Quality Control Standards for Polyphenolic Compounds in Supplement Manufacturing." Analytical Chemistry in Food Science, vol. 67, no. 4, 2020, pp. 203-218.



